Vaccines used during the smallpox eradication programme also provided protection against monkeypox. Newer vaccines have been developed of which one has been approved for prevention of monkeypox
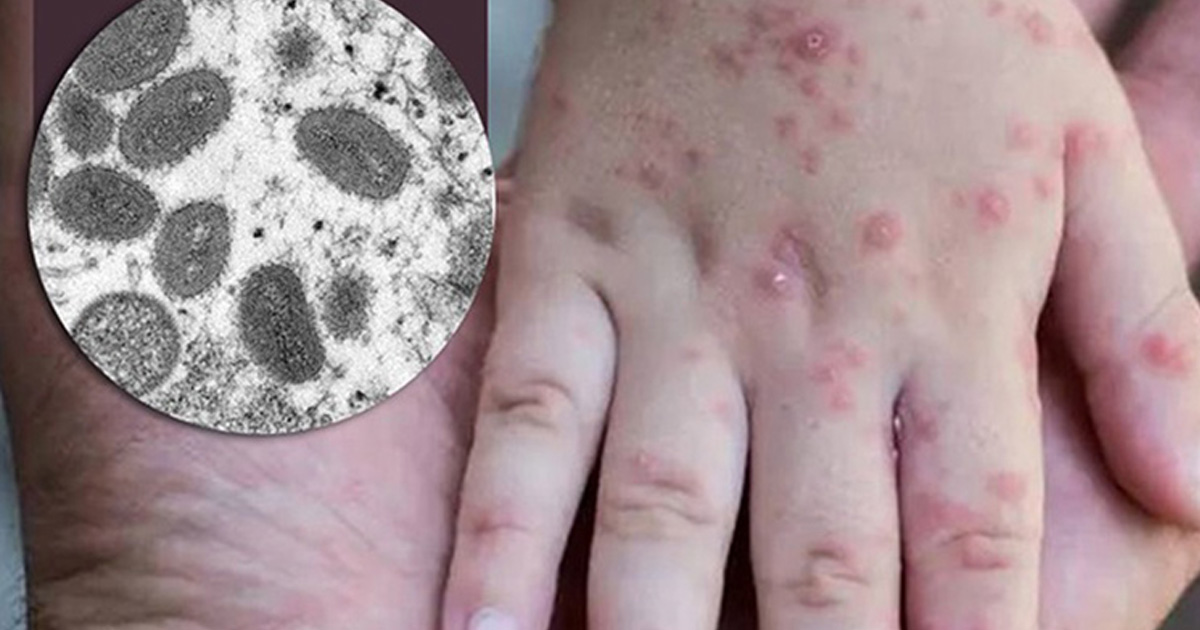
Monkeypox is caused by monkeypox virus, a member of the Orthopoxvirus genus in the family Poxviridae.
Monkeypox is usually a self-limited disease with the symptoms lasting from 2 to 4 weeks. Severe cases can occur. In recent times, the case fatality ratio has been around 3–6%.
Monkeypox is transmitted to humans through close contact with an infected person or animal, or with material contaminated with the virus.
Monkeypox virus is transmitted from one person to another by close contact with lesions, body fluids, respiratory droplets and contaminated materials such as bedding.
Monkeypox is a viral zoonotic disease that occurs primarily in tropical rainforest areas of central and west Africa and is occasionally exported to other regions.
An antiviral agent developed for the treatment of smallpox has also been licensed for the treatment of monkeypox.
The clinical presentation of monkeypox resembles that of smallpox, a related orthopoxvirus infection which was declared eradicated worldwide in 1980. Monkeypox is less contagious than smallpox and causes less severe illness.
Monkeypox typically presents clinically with fever, rash and swollen lymph nodes and may lead to a range of medical complications.

Introduction
Monkeypox is a viral zoonosis (a virus transmitted to humans from animals) with symptoms similar to those seen in the past in smallpox patients, although it is clinically less severe. With the eradication of smallpox in 1980 and subsequent cessation of smallpox vaccination, monkeypox has emerged as the most important orthopoxvirus for public health. Monkeypox primarily occurs in central and west Africa, often in proximity to tropical rainforests, and has been increasingly appearing in urban areas. Animal hosts include a range of rodents and non-human primates.
The pathogen
Monkeypox virus is an enveloped double-stranded DNA virus that belongs to the Orthopoxvirus genus of the Poxviridae family. There are two distinct genetic clades of the monkeypox virus: the central African (Congo Basin) clade and the west African clade. The Congo Basin clade has historically caused more severe disease and was thought to be more transmissible. The geographical division between the two clades has so far been in Cameroon, the only country where both virus clades have been found.
Natural host of monkeypox virus
Various animal species have been identified as susceptible to monkeypox virus. This includes rope squirrels, tree squirrels, Gambian pouched rats, dormice, non-human primates and other species. Uncertainty remains on the natural history of monkeypox virus and further studies are needed to identify the exact reservoir(s) and how virus circulation is maintained in nature.
Outbreaks
Human monkeypox was first identified in humans in 1970 in the Democratic Republic of the Congo in a 9-year-old boy in a region where smallpox had been eliminated in 1968. Since then, most cases have been reported from rural, rainforest regions of the Congo Basin, particularly in the Democratic Republic of the Congo and human cases have increasingly been reported from across central and west Africa.
Since 1970, human cases of monkeypox have been reported in 11 African countries: Benin, Cameroon, the Central African Republic, the Democratic Republic of the Congo, Gabon, Cote d’Ivoire, Liberia, Nigeria, the Republic of the Congo, Sierra Leone and South Sudan. The true burden of monkeypox is not known. For example, in 1996–97, an outbreak was reported in the Democratic Republic of the Congo with a lower case fatality ratio and a higher attack rate than usual. A concurrent outbreak of chickenpox (caused by the varicella virus, which is not an orthopoxvirus) andmonkeypox was found, which could explain real or apparent changes in transmission dynamics in this case. Since 2017, Nigeria has experienced a large outbreak, with over 500 suspected cases and over 200 confirmed cases and a case fatality ratio of approximately 3%. Cases continue to be reported until today.
Monkeypox is a disease of global public health importance as it not only affects countries in west and central Africa, but the rest of the world. In 2003, the first monkeypox outbreak outside of Africa was in the United States of America and was linked to contact with infected pet prairie dogs. These pets had been housed with Gambian pouched rats and dormice that had been imported into the country from Ghana. This outbreak led to over 70 cases of monkeypox in the U.S. Monkeypox has also been reported in travelers from Nigeria to Israel in September 2018, to the United Kingdom in September 2018, December 2019, May 2021 and May 2022, to Singapore in May 2019, and to the United States of America in July and November 2021. In May 2022, multiple cases of monkeypox were identified in several non-endemic countries. Studies are currently underway to further understand the epidemiology, sources of infection, and transmission patterns.
Transmission
Animal-to-human (zoonotic) transmission can occur from direct contact with the blood, bodily fluids, or cutaneous or mucosal lesions of infected animals. In Africa, evidence of monkeypox virus infection has been found in many animals including rope squirrels, tree squirrels, Gambian poached rats, dormice, different species of monkeys and others. The natural reservoir of monkeypox has not yet been identified, though rodents are the most likely. Eating inadequately cooked meat and other animal products of infected animals is a possible risk factor. People living in or near forested areas may have indirect or low-level exposure to infected animals.
Human-to-human transmission can result from close contact with respiratory secretions, skin lesions of an infected person or recently contaminated objects. Transmission via droplet respiratory particles usually requires prolonged face-to-face contact, which puts health workers, household members and other close contacts of active cases at greater risk. However, the longest documented chain of transmission in a community has risen in recent years from 6 to 9 successive person-to-person infections. This may reflect declining immunity in all communities due to cessation of smallpox vaccination. Transmission can also occur via the placenta from mother to fetus (which can lead to congenital monkeypox) or during close contact during and after birth. While close physical contact is a well-known risk factor for transmission, it is unclear at this time if monkeypox can be transmitted specifically through sexual transmission routes. Studies are needed to better understand this risk.
Signs and symptoms
The incubation period (interval from infection to onset of symptoms) of monkeypox is usually from 6 to 13 days but can range from 5 to 21 days.
The infection can be divided into two periods:
the invasion period (lasts between 0–5 days) characterized by fever, intense headache, lymphadenopathy (swelling of the lymph nodes), back pain, myalgia (muscle aches) and intense asthenia (lack of energy). Lymphadenopathy is a distinctive feature of monkeypox compared to other diseases that may initially appear similar (chickenpox, measles, smallpox)
the skin eruption usually begins within 1–3 days of appearance of fever. The rash tends to be more concentrated on the face and extremities rather than on the trunk. It affects the face (in 95% of cases), and palms of the hands and soles of the feet (in 75% of cases). Also affected are oral mucous membranes (in 70% of cases), genitalia (30%), and conjunctivae (20%), as well as the cornea. The rash evolves sequentially from macules (lesions with a flat base) to papules (slightly raised firm lesions), vesicles (lesions filled with clear fluid), pustules (lesions filled with yellowish fluid), and crusts which dry up and fall off. The number of lesions varies from a few to several thousand. In severe cases, lesions can coalesce until large sections of skin slough off.
Monkeypox is usually a self-limited disease with the symptoms lasting from 2 to 4 weeks. Severe cases occur more commonly among children and are related to the extent of virus exposure, patient health status and nature of complications. Underlying immune deficiencies may lead to worse outcomes. Although vaccination against smallpox was protective in the past, today persons younger than 40 to 50 years of age (depending on the country) may be more susceptible to monkeypox due to cessation of smallpox vaccination campaigns globally after eradication of the disease. Complications of monkeypox can include secondary infections, bronchopneumonia, sepsis, encephalitis, and infection of the cornea with ensuing loss of vision. The extent to which asymptomatic infection may occur is unknown.
The case fatality ratio of monkeypox has historically ranged from 0 to 11 % in the general population and has been higher among young children. In recent times, the case fatality ratio has been around 3–6%.

Diagnosis
The clinical differential diagnosis that must be considered includes other rash illnesses, such as chickenpox, measles, bacterial skin infections, scabies, syphilis, and medication-associated allergies. Lymphadenopathy during the prodromal stage of illness can be a clinical feature to distinguish monkeypox from chickenpox or smallpox.
If monkeypox is suspected, health workers should collect an appropriate sample and have it transported safely to a laboratory with appropriate capability. Confirmation of monkeypox depends on the type and quality of the specimen and the type of laboratory test. Thus, specimens should be packaged and shipped in accordance with national and international requirements. Polymerase chain reaction (PCR) is the preferred laboratory test given its accuracy and sensitivity. For this, optimal diagnostic samples for monkeypox are from skin lesions – the roof or fluid from vesicles and pustules, and dry crusts. Where feasible, biopsy is an option. Lesion samples must be stored in a dry, sterile tube (no viral transport media) and kept cold. PCR blood tests are usually inconclusive because of the short duration of viremia relative to the timing of specimen collection after symptoms begin and should not be routinely collected from patients.
As orthopoxviruses are serologically cross-reactive, antigen and antibody detection methods do not provide monkeypox-specific confirmation. Serology and antigen detection methods are therefore not recommended for diagnosis or case investigation where resources are limited. Additionally, recent or remote vaccination with a vaccinia-based vaccine (e.g. anyone vaccinated before smallpox eradication, or more recently vaccinated due to higher risk such as orthopoxvirus laboratory personnel) might lead to false positive results.
In order to interpret test results, it is critical that patient information be provided with the specimens including: a) date of onset of fever, b) date of onset of rash, c) date of specimen collection, d) current status of the individual (stage of rash), and e) age.
Therapeutics
Clinical care for monkeypox should be fully optimized to alleviate symptoms, manage complications and prevent long-term sequelae. Patients should be offered fluids and food to maintain adequate nutritional status. Secondary bacterial infections should be treated as indicated. An antiviral agent known as tecovirimat that was developed for smallpox was licensed by the European Medical Association (EMA) for monkeypox in 2022 based on data in animal and human studies. It is not yet widely available.
If used for patient care, tecovirimat should ideally be monitored in a clinical research context with prospective data collection.

Vaccination
Vaccination against smallpox was demonstrated through several observational studies to be about 85% effective in preventing monkeypox. Thus, prior smallpox vaccination may result in milder illness. Evidence of prior vaccination against smallpox can usually be found as a scar on the upper arm. At the present time, the original (first-generation) smallpox vaccines are no longer available to the general public. Some laboratory personnel or health workers may have received a more recent smallpox vaccine to protect them in the event of exposure to orthopoxviruses in the workplace. A still newer vaccine based on a modified attenuated vaccinia virus (Ankara strain) was approved for the prevention of monkeypox in 2019. This is a two-dose vaccine for which availability remains limited. Smallpox and monkeypox vaccines are developed in formulations based on the vaccinia virus due to cross-protection afforded for the immune response to orthopoxviruses.
Prevention
Raising awareness of risk factors and educating people about the measures they can take to reduce exposure to the virus is the main prevention strategy for monkeypox. Scientific studies are now underway to assess the feasibility and appropriateness of vaccination for the prevention and control of monkeypox. Some countries have, or are developing, policies to offer vaccine to persons who may be at risk such as laboratory personnel, rapid response teams and health workers.